To estimate the cost of building a website or an app, use our app cost calculator tool.
If a healthcare application touches patient data or supports clinical decision-making, the FDA regulates it. Many early-stage startups don’t realize this until compliance issues delay launches or force expensive rework.
The industry data is a wake-up call. According to recent FDA 510(k) submission trends, over 65% of applications are initially rejected or placed on hold because of missing documentation or compliance gaps. These aren't edge cases; they are the result of fast-moving teams shipping code without a regulatory plan.
Startups that treat FDA compliance as something to "fix later" are actually slowing themselves down. Conversely, teams that build compliance into their development process from day one reduce risk, avoid the "re-work trap," and keep their momentum.
In this article, we’ll simplify what FDA compliance actually means for startups and walk through how to build compliant software without sacrificing speed.
Let’s get started.
Understanding FDA Compliance in Software Development
1. What FDA compliance actually means for software (not hardware)
When you think of FDA compliance, two aspects come up. One aspect pertains to medical devices with physical components, and the other to software; compliance encompasses both as they impact patient health.
There are two broad categories, and we will look at them.
- Software as a Medical Device (SaMD)
In this context, software that has the capacity to perform a medical function independently, such as the Apple Watch ECG app, which can detect atrial fibrillation, falls under this category.
For example, diagnostic apps and AI-driven imaging software.
- Software in a Medical Device (SiMD)
In this case, all software that is part of a hardware device, which could be either controlling or supporting its function, will be in this category.
For example, insulin pump firmware.
The FDA focuses on three primary priorities:
- Safety: Does the software avoid causing harm?
- Effectiveness: When it comes to its intended medical purpose, does it operate reliably?
- Risk management: Are potential hazards spotted, reduced, and monitored?
2. The 4 compliance layers founders often overlook
Most digital health founders don’t ignore compliance on purpose. It usually slips through the cracks because early teams are focused on shipping fast, raising capital, and validating the product. But missing these foundational compliance layers can lead to delays, rework, or even regulatory violations later on.
Here are the four areas startups most commonly underestimate.
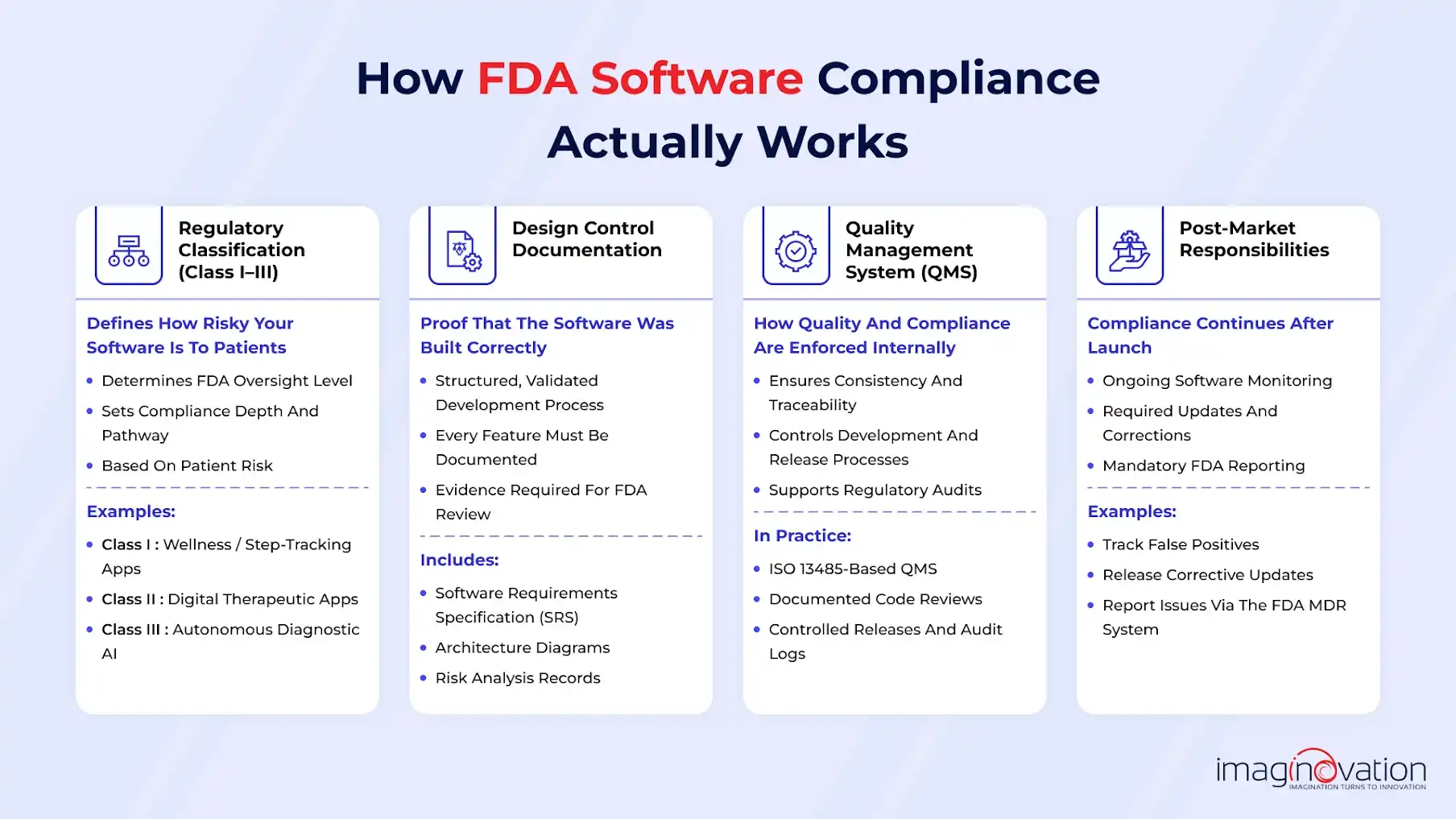
a) Regulatory classification (Class I–III)
The FDA classifies medical devices, including software, into three categories based on patient risk. This classification determines how much regulatory oversight your product requires.
For example, basic wellness apps like step trackers are typically considered low risk and fall under Class I.
Digital therapeutics that guide treatment decisions often land in Class II, which comes with more regulatory requirements.
At the high end, fully autonomous diagnostic AI systems are usually classified as Class III, where FDA scrutiny is the highest.
Getting this classification right early shapes everything that follows.
b) Design control documentation
FDA compliance is not just about what your software does. It’s also about proving how it was built.
That means keeping clear, organized documentation that shows your product was developed using controlled, validated processes. This includes documenting each feature and development step, from your Software Requirements Specification (SRS) to architecture diagrams and risk analysis records.
Good documentation makes compliance easier to demonstrate and far less painful during reviews.
c) Quality Management System (QMS)
A Quality Management System helps ensure your internal processes stay consistent as your product and team scale. It creates structure around quality, traceability, and regulatory accountability.
For example, a digital health startup might implement an ISO 13485–aligned QMS that includes documented code reviews, controlled release processes, and audit logs for every software update.
This kind of system builds trust with regulators and reduces risk as development accelerates.
d) Post-market responsibilities
FDA obligations don’t stop once your product launches.
After release, startups are responsible for monitoring performance, addressing issues, and reporting certain events.
For instance, an AI diagnostic app may track false positives, release corrective updates when issues arise, and submit required reports through the FDA’s Medical Device Reporting (MDR) system. Ongoing oversight is part of staying compliant long term.
💡 According to Pete Peranzo, Co-founder of Imaginovation, the most deeply ingrained myth that startups have about FDA compliance is that compliance comes later, after development.
He shares that this misconception often costs them because they fail to understand or implement FDA compliance, or other health-related compliances like HIPAA, before they start developing the software.
Startups, being new in the market, often focus on the idea and building the product first. This leads them to push regulatory compliance to a later stage, which is a misplaced timing that can cause expensive regulatory mistakes that later need to be fixed post-development.
Key Takeaway
FDA expectations for software are just as strict as they are for traditional medical devices, especially for AI-driven diagnostics and treatment tools.
For digital health startups, this means demonstrating structured development practices, clear documentation, and ongoing monitoring, since even small software updates can change clinical risk.
Do You Even Need FDA Compliance?
Many new founders often misjudge whether their product falls under FDA oversight.
It is an interesting observation that while some overestimate the need for compliance and overspend months (and lakhs) on unnecessary documentation.
Others ignore it entirely, which could lead to blocked launches. Moreover, it exposes them to warnings or even takedown notices.
The truth is not every wellness or tracking app requires FDA clearance. Founders must explore what the FDA actually regulates, which acts as the key to working around the compliance requirements.
1. Decision framework
Here’s a quick snapshot that will help you understand if you need FDA compliance.
Table 1: Which App Requires FDA Oversight?
| Type of App | Function | FDA-Regulated? | Example |
|---|---|---|---|
| Step counter | Tracks steps only | ❌ No | Fitbit (basic step-tracking mode) |
| Sleep tracker | Logs patterns, no diagnosis | ❌ No | Oura (sleep stages only) |
| Symptom journaling app | User self-input, no clinical claims | ❌ No | Migraine diary |
| Glucose tracker using sensor data | Measures real health metrics | ✅ Yes | Dexcom |
| App adjusting insulin dosage | Provides treatment recommendations | ✅ Yes (High-risk) | Insulin dosing calculator |
| AI skin-lesion analyzer | Gives diagnostic suggestions | ✅ Yes (High-risk) | Mole-check AI |
2. How to self-assess risk early
For self-assessing FDA risk early, you can examine what your software actually does.
The FDA's "Intended Use" test is based on software functionality on aspects of how it measures, analyzes, or influences health decisions.
If your software processes:
- Physiological signals
- Interprets sensor data
- Triggers clinical-like alerts
- Offers diagnostic or treatment-related guidance
- Interacts with a medical device
It likely qualifies as SaMD and will require FDA oversight.
However, if it is simply:
- Tracks habits
- Logs user-entered data
- Visualizes information without interpretation
- Offers general wellness nudges
It typically remains non-regulated.
At times, the best approach is to consult a regulatory specialist before locking in technical decisions that could increase risk or may require compliance rework later.
Consulting is vital, especially if the software will:
- Integrate with medical sensors
- Use AI/ML to infer health conditions
- Provide clinical decision support
- Drive therapeutic actions
- Interface with regulated devices or EHR systems
The expert input when sought early ensures your architecture, data flows, and model design meet compliance expectations. The insights can help avoid expensive redesigns and delays once development is underway.
Key takeaway
Consider assessing the various functionalities of your software early. Getting expert consultation can save you from costly redesigns and regulatory delays.
The FDA Compliance Roadmap for Startups
Step 1: Regulatory Discovery
The founders can start by mapping the intended medical use of the software. For now, it's inevitable that your product either falls under SaMD or the software functions as an accessory to a medical device.
From there, based on insight from the classification, draft a preliminary classification rationale that will help understand the regulatory pathway.
For example, a glucose-tracking app is considered SaMD because it informs medical decisions, whereas a simple step-counter app falls under general wellness.
Step 2: Build Compliance Into Design
Once the foundation is laid, it's time to embed design controls into agile workflows.
Remember to keep DHFs and traceability matrices, even for MVPs. It will be a great idea to run validation sprints that test features against risk profiles.
Example: While building an insulin-dosing feature, maintain traceability from requirements to code to tests.
Step 3: Documentation, Validation, and Submission
Post-design state, it is time to prepare core deliverables for 510(k), De Novo, or PMA pathways. You can build review-ready documentation alongside coding.
It is also time to focus on using a structured design dossier for audits and regulatory review.
Example: A design dossier capturing code, tests, risk assessment, and clinical rationale for a 510(k) submission.
Step 4: Post-Market Vigilance
The post-market stage is the most crucial stage; now is the time to find bugs and take immediate action upon them, as they can potentially be reportable events.
Remember, version control and update management are a must. You can also embed compliance into DevOps and CI/CD pipelines.
Example: A crash in a heart-rate monitoring app may trigger a reportable event; the CI/CD pipelines track the versions and fixes.
💡 In this context, Pete Peranzo, Co-founder of Imaginovation shares that the best approach is to treat FDA compliance as a lightweight backbone.
Pete adds that startup founders can build MVP fast, and then track requirements to code and validate critical workflows. He shares that the approach will keep you agile and help you stay on the right side of regulation.
Key Takeaway
Founders can treat FDA compliance as a parallel track to development and classify early. It is also essential to focus on documenting as you code and monitoring after launch to avoid surprises and accelerate approvals.
The Cost of Getting It Wrong
Let's explore the spots where you can have a misstep. An early find can help to look out for such traps and preempt them to fix them.
Common Regulatory Traps
1. Launching a “wellness” app that makes diagnostic claims.
Example: A sleep app that says “screens for sleep apnea” instantly crosses into regulated territory and triggers FDA scrutiny.
2. Ignoring design documentation until after launch.
Example: This is a classic case where teams try to reconstruct user needs, risk logs, and validation evidence months later, which is usually impossible and often results in rejection.
3. Using unvalidated AI/ML models.
Example: A symptom-checker trained on biased datasets misclassifies risk for certain demographics, raising safety and ethics concerns for the startup.
Hidden Ripple Effects
1. When a startup founder can't explain how their product will be regulated by the FDA, the investors may pull back due to this regulatory uncertainty.
Example: A Venture Capitalist delays the term sheet because the team can’t clearly articulate the regulatory pathway or classification.
2. Consider an app store delisting or mandatory recall.
Example: After a consumer complaint, Apple removes the app for “unverified medical advice,” forcing an urgent relaunch.
3. Some founders may have burnt runway, leading to loss of brand trust.
Example: Retrofitting traceability or redesigning the algorithm for compliance consumes 4-6 months of runway that early-stage teams can’t afford.
Quick-Win Prevention Steps
1. It is a great idea to run compliance audits every sprint. For instance, even a 10-minute claims check reduces accidental regulatory drift.
2. Yet another aspect to incorporate is to keep a “living” risk-management file. The file or document, in simple words, gets continually updated.
Thus, every time you build or release a new feature, add risks and mitigations for each new feature. You will start seeing better results when you aren't waiting for the end.
3. It is vital to engage early with FDA pre-submission programs. For instance, a short pre-sub discussion can correct wrong assumptions about classification or data requirements.
Key Takeaway
Early missteps with FDA compliance don’t just slow approvals, but they can affect at a greater level with funding, user trust, speed to market, and survival. A few lightweight safeguards can prevent months of rework and protect a startup’s runway.
Choosing the Right FDA-Compliant Development Partner
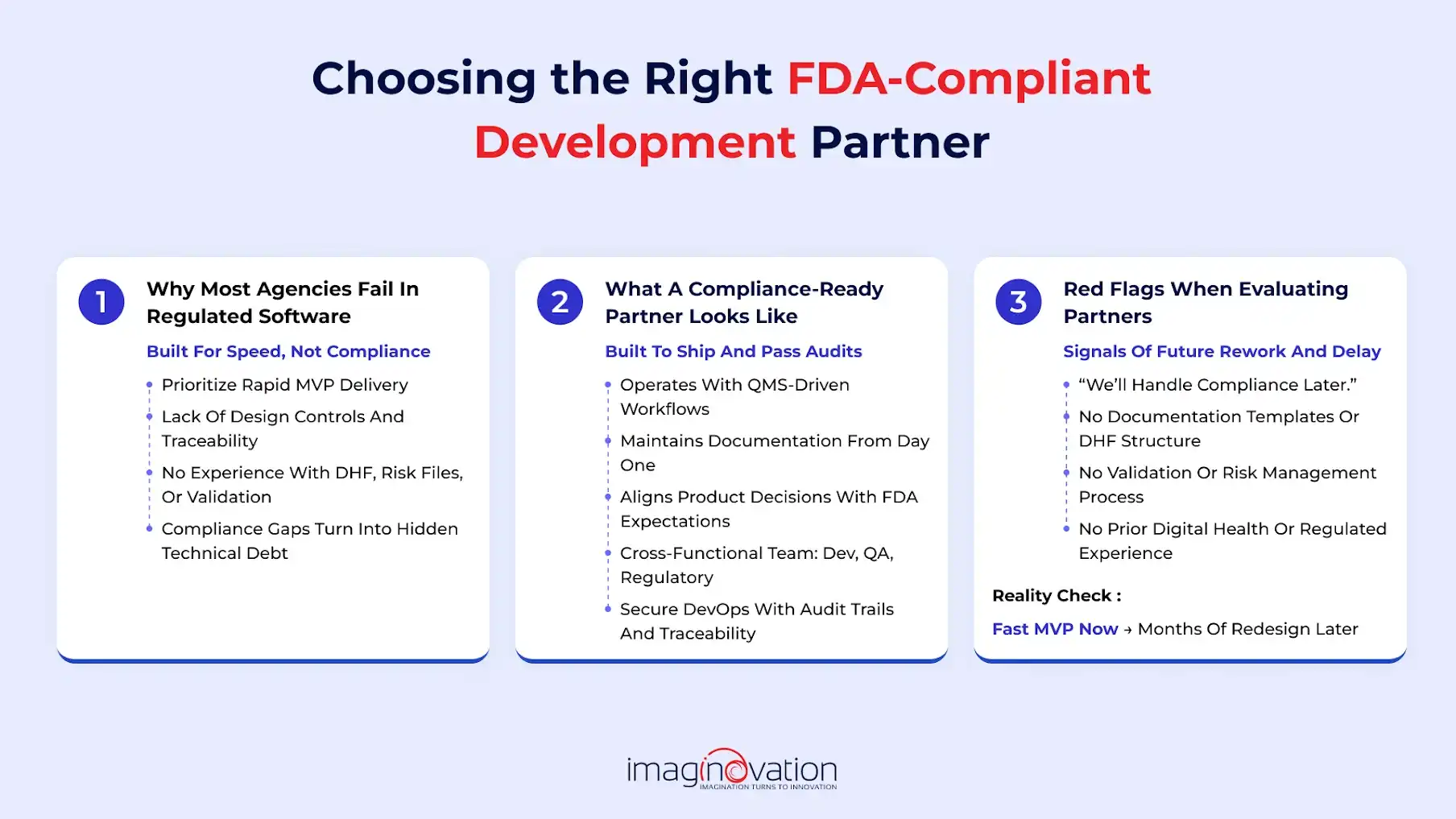
When it comes to agencies, quite often they are built to maximize speed, and quite a few find it challenging to maintain the documentation and traceability required in regulated software.
They rarely work with design controls, DHFs, risk files, or validation protocols. This backdrop unknowingly pushes startups toward hidden compliance debt.
What a true compliance-ready partner looks like
In such scenarios, startup founders may want to look out for a partner who has a proven track record of compliance readiness.
Why?
A compliance-ready partner operates with proven QMS-driven workflows. They can help bring structure without slowing product development.
Moreover, their team can offer expert insight, and their developers, QA engineers, and regulatory advisors can ensure that product decisions are totally aligned with FDA expectations.
They can also run secure DevOps pipelines with audit trails, so every build is automatically traceable and evidence-ready.
Red flags when evaluating partners
Here are some scenarios you may have encountered:
- “We’ll worry about compliance later.”
- No documentation templates, DHF structure, or validation processes.
- Lack of experience in digital health or regulated industries.
These are signs the partner may get you an MVP fast, but could cost you months of redesign later.
How Imaginovation supports startups
Imaginovation helps startups stay FDA-aware from day zero through early risk discovery sessions that clarify intended use, features with regulatory impact, and potential classification before development starts.
Their validation-driven agile framework integrates design controls, testing, and documentation into every sprint, so startups can ship quickly without regulatory drift.
With a strong track record in regulated SaaS and healthtech, they know how to balance agility with compliance discipline.
💡 A recent example is their collaboration on Everflex, a custom healthcare platform built for a growing physical-therapy network. The founders needed to scale quickly but safely.
Imaginovation began with deep workflow and risk discovery, then paired each sprint with validation, security checks, and traceability, practices essential for regulated environments.
The final platform cut software complexity nearly in half and improved patient engagement significantly, demonstrating how the right partner helps startups grow fast without compromising compliance.
Key Takeaway
Choosing a compliance-ready partner early can save your startup months of rework, preserve runway, and accelerate a safer, smoother path to market.
The Future of FDA-Compliant Software
The FDA is no longer static and is far from it, and is increasingly relying on one-time approvals toward a more comprehensive total product lifecycle framework.
For AI/ML systems that are changing and, more importantly, improving over time, regulators in that context now require:
- Performance monitoring as a continual process, such that it can help track real-world behavior
- Defined boundaries for acceptable model updates
- Processes that are well-defined and clear for managing data quality, and can handle model drift and emerging risks
The shift helps balance innovation with rigorous safety standards, creating space for both and thereby increasing effectiveness throughout a product's lifetime.
The New "Predetermined Change Control Plan" (PCCP) - What It Means for Startups
The PCCP represents a fundamental shift in regulatory thinking. It enables companies to establish upfront:
- Which model modifications do they anticipate making
- The testing and validation protocols for those changes
- Their approach to monitoring real-world performance
For startups, a thoughtfully designed PCCP becomes a competitive asset.
It can take care of any regulatory submissions that happen next, reducing expensive rework cycles and ensuring that your product roadmap and regulatory strategy are aligned from the start.
Why Compliance Automation Tools Are Emerging and How Founders Can Stay Ahead
Lifecycle-based regulation demands a scalable compliance infrastructure. A new generation of automation tools is helping startups manage:
- Complete audit trails and regulatory documentation
- Automated detection of model drift with integrated validation workflows
- Evidence packages ready for regulatory submissions
- Full traceability connecting data sources, design decisions, and test results
Founders who invest in these capabilities early gain significant advantages: faster development cycles, stronger regulatory confidence, and the ability to build adaptive AI products with smaller teams while maintaining rigorous safety standards.
Wrapping Up
FDA compliance isn’t a blocker. It’s how digital health startups move faster and scale with confidence.
Founders who plan early by defining claims, choosing the right classification, and building design controls into development avoid costly rework later.
Imaginovation helps digital health and wellness startups build FDA-compliant software from the ground up, from architecture decisions to regulatory submissions. We support teams across complex areas, including PCCP strategies that stand up to scrutiny.
If your software touches patient care and you want to reach the market without cutting corners, let’s talk.